The tubular flow reactor is a simple device that allows for the continuous generation of reaction products. It is comprised of a long tube in which reactants are fed in one end and flow down the entire length. Products are then collected at the opposite end, as shown in Figure 1. Conductivity probes, which measure the strength of electrolytes, may be placed near the entrance and exit of the reactor to continuously monitor the progress of the reaction.
Figure 1  Schematic of the Tubular Flow Reactor
Schematic of the Tubular Flow Reactor
The saponification of ethyl acetate (EtAc) may be used with this particular reactor, and is given by the irreversible reaction:
CH3CH2COOCH3 + NaOH 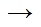 CH3CH2OH + NaCOOCH3
CH3CH2OH + NaCOOCH3
Separate tanks of CH3CH2COOCH3 and NaOH are fed to the entrance of the reactor in such a way that NaOH is in excess. To measure the concentration of NaOH entering the reactor, a preparation of acidic potassium hydrogen phthalate (KHP) is titrated with the NaOH (sampled from the feed tank) until a phenolphthalein end point. Phenolphthalein is an indicator that is colorless in acidic solutions but turns purple after a large shift toward basic pH.
Since EtAc and ethanol are not electrically conductive, preliminary conductivity measurements on the other two species were performed and shown in Figure 2.
Figure 2
In designing a reactor, important parameters to consider include the flow rate and the reactor length. These parameters control the residence time, or actual time it takes the reactants to flow from one end of the reactor to the other. Longer residence times increase the probability that the reaction will go to completion.